Exotic Chemical Thread
#46

Posted 27 October 2009 - 07:28 AM
Now that's interesting. I can get a whole bunch of it, but in my experience once mixed in a compo it gets very hygroscopical. And this IS the species that give blue in flame (actually Cu2Cl2).
Maybe someone has some formulas using it?
What else can I get out of it? I was thinking about turning it into copper oxychloride. Will in this case work just a mix with hydrogen peroxide?
#47

Posted 27 October 2009 - 10:37 AM
Potassium perchlorate.............................65
Cuprous chloride (CuCl)...........................16
Sulfur............................................10
Red gum...........................................7
Parlon or PVC.....................................11 or 12
used for sundry preparations, and especially for experimental
fire-works."
Dr. James Cutbush
#48

Posted 27 October 2009 - 12:16 PM
I don't think cuperous chloride (cucl) is hygroscopic i have discovered a good way to make this using cuso4,koh,and hcl and have produced some good blues in the tests i have done. Copper chloride (cucl2) is very hygroscopic and is only really any good in flame boxes.
I could try and make some strontium perchlorate with perchloric acid and strontium carbonate but i do believe it would be very hygroscopic.
#49

Posted 27 October 2009 - 12:48 PM
Nevertheless, somewhere on the net there were some formulas under the name of "russian fires" or something. They were all pretty much based on these perchlorates (Ba and Sr) and the results were impressive. Sort of like using barium chlorate: a molecule with both the chlorine AND the colorant metal.
Edited by a_bab, 27 October 2009 - 12:51 PM.
#50

Posted 27 October 2009 - 01:14 PM
Edited by Pyroswede, 27 October 2009 - 01:14 PM.
used for sundry preparations, and especially for experimental
fire-works."
Dr. James Cutbush
#51

Posted 27 October 2009 - 02:37 PM
How exactly does the reaction of Copper (II) Sulfate, KOH and HCl produce Cu (I) Cl? Unless you're making SO2 or Cl2 somehow insitu, I don't see how it can happen.
#52

Posted 27 October 2009 - 03:29 PM
When you disolve cupric hydroxide in hcl you make cupric chloride(soluble in water) by treating this with metallic copper you make cuprous chloride (insoluble in water)
#53

Posted 27 October 2009 - 08:51 PM
My question: how can I turn say 1 kg of CuCl(I) to copper oxychloride?
#54

Posted 27 October 2009 - 10:27 PM
The disoloration of CuCl is from disproportionation to Cu and CuCl2, as well as formation of carbonates and oxychloride.
To convert to the oxychloride just bubble air through it for a while. The oxygen should oxidize it back to Copper (II). Dissolving it in HCl might help increase the reaction rate. Cu2Cl2 is soluble in HCl forming CuCl2(-). Alternatively a 1:1 molar ratio of CuO and CuCl2 is boiled for several hours, filtered, and washed with acetone. I think the usual method. Adding a 1/2 molar equivalent of KOH to CuCl2 and boiling should do the trick too.
Two new compounds I came upon while looking up syntheses. KCuO2, yes potassium cuprate. It seems to be quite a vigorous oxizider. Very hard to make, and very reactive so probably nothing more than a theoretical interest. It's formed by fusing CuO and KOx. Finally a copper based oxidizer. It decomposes in water and acid. I wouldn't be putting Copper (III) near any conventional solvents either.
Now a realistic compound, copper (I) Bromide. Again the emitter is CuBr, and the compound is Cu2Br2. It gives a strong pure blue line, and a purple line. It may form a very nice color. There are a few commonly available polychlorinated bromine compounds being used as anti-flame agents in plastics that could serve as bromine donors. Time for some samples for a newly founded plastic manufacturer
#55

Posted 28 October 2009 - 10:29 PM
This bromine thing sounds interesting i've often wondered if it would act like chlorine at temperature in fact i'm awaiting my bromine delivery,i'll keep you posted
#56

Posted 30 October 2009 - 07:22 PM
I have personally tried calcium, barium and copper fluoride to little benefit. Calcium fluoride does still produce a very pure pink-red.
#57

Posted 30 October 2009 - 08:37 PM
#58

Posted 30 October 2009 - 10:34 PM
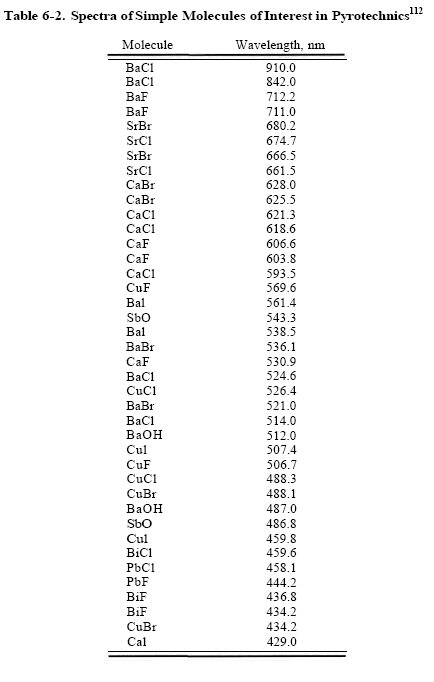
#59

Posted 31 October 2009 - 12:32 AM
#60

Posted 01 November 2009 - 11:39 PM
The same emitters appear more than once because they emit multiple lines. There is no such thing as a monochromatic chemical emitter or formula. They do not emit bands of light, rather discrete wavelengths. For instance, BaBr emits at 536.1nm and 521.1nm, but not in between them.
1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users













